Pequeno Príncipe carries out clinical trials with new medication
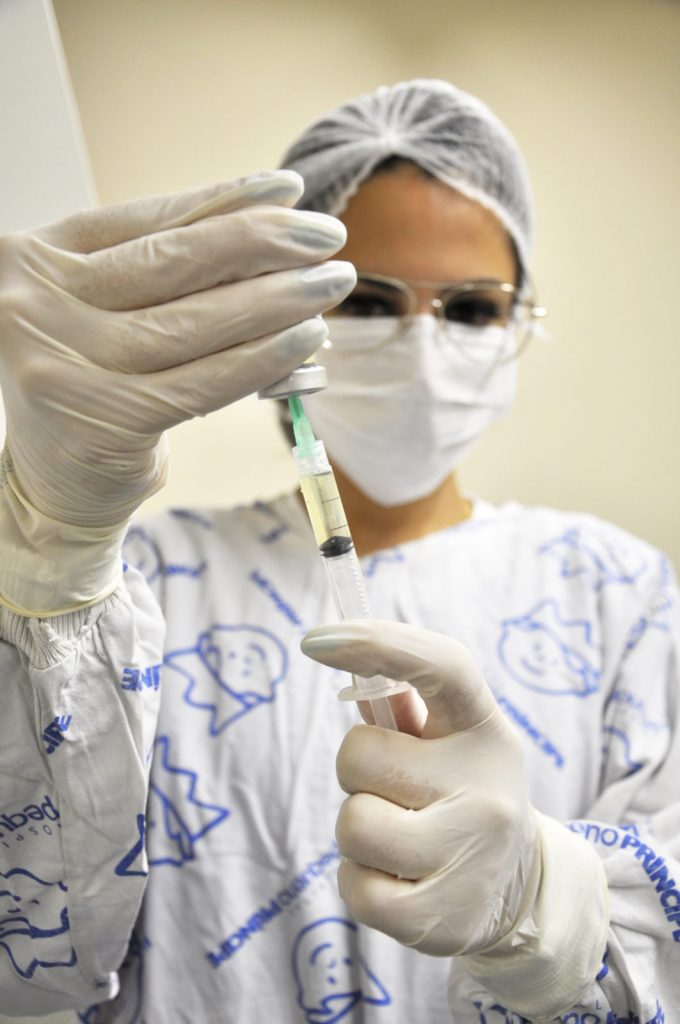 Investment in clinical trials is one of the paths to find new treatments to cure or improve the quality of life of children and teenagers affected by complex diseases.
Investment in clinical trials is one of the paths to find new treatments to cure or improve the quality of life of children and teenagers affected by complex diseases.
Pequeno Príncipe Hospital’s Clinical Research Center coordinates research trials in partnership with the pharmaceutical industry, to develop and approve new medication in Pediatrics.
Marinei Campos Ricieri, coordinator of the Clinical Research Center, explains: “These research projects can provide pediatric patients with new treatment options for diseases where regulated medicated alternatives for children are scarce and, depending on the disease, nonexistent.”
About 15 clinical trials were carried out in Pequeno Príncipe in 2017, with focus in the fields of Infectology and Rheumatology.
These clinical trials were approved by the National Ethics in Research Committee (CONEP) and by the Pequeno Príncipe Ethics in Research Committee (CEP). The families and children above seven years old that participated in the study were previously consulted about their wish to participate in the research and approved their participation by signing a consent form.
 Research projects in Brazil and the world
Research projects in Brazil and the world
Brazil is currently placed in 13th in the world ranking of records of clinical trials, a market led by the United States, Germany and France.
The challenge is even greater when considering clinical research in Pediatrics. For example, about 80% of medication that has been approved in the United States aren’t for pediatric use and contains incomplete information about the effects it could cause in children. In Europe, about 50% of medication used in children didn’t pass through research trials or specific authorization for pediatric use, categorizing it as off label use.
